Sir Ernest Rutherford

In 1909 Rutherford directed the Geiger – Marsden experiment. In this experiment alpha particles were directed at a thin gold foil and the scattering pattern was studied. Most of the alpha particles passed strait through the gold foil, some were deflected at small angels and some were deflected at very large angles.
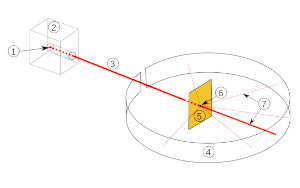
Rutherford scattering experiment
wikimedia open source
In 1911 Rutherford published his theory stating in order to explain the very large deflection angles most of the mass and the positive charge of the atom must be small and located at the center of the atom. The electrons making up a sphere of negative charge around the perimeter of the atom.
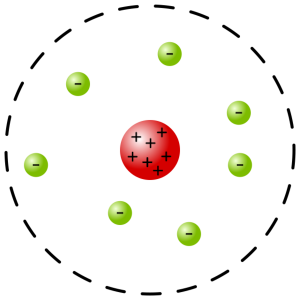
Rutherford planetary model
Creative Commons Attribution-Share Alike 3.0
The thing is, this model could not be reconciled with Coulomb’s inverse square law. Negative charges placed statically near a positive charge would not be stable. Angular momentum (electrons orbiting around the nucleus like planets orbiting the sun) could balance the electrostatic force of attraction, but an orbiting charge was known to emit radiation. The emitted radiation would result in a loss of energy resulting in the electron spiraling down into the nucleus.
After 126 years Coulomb’s inverse square law continued to have a strong influence on our understanding of the atom.