Niels Bohr

In 1913 Neils Bohr proposed the Bohr Atomic Model. The Bohr model for the hydrogen atom was composed of a central nucleus with a single positive charge and one electron orbiting the nucleus with sufficient velocity so that the angular momentum would balance out the electrostatic force of attraction.
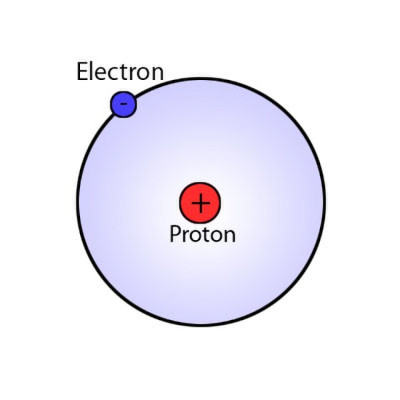
Bohr model of the atom
Now classically, since the electron is accelerated, this system should radiate (this was Rutherford’s stumbling block). To avoid this difficulty, Bohr broke with tradition (IE classical physics).
Bohr assumed:
1. The electron can move around the nucleus only in certain orbits, and not in others (classically, no particular orbit was preferred).
2. These allowed orbits correspond to definite stationary states of the atom, and in such a stationary state the atom is stable and does not radiate.
3. The electron emits or adsorbs only certain amounts of energy when transitioning from one energy state to another.
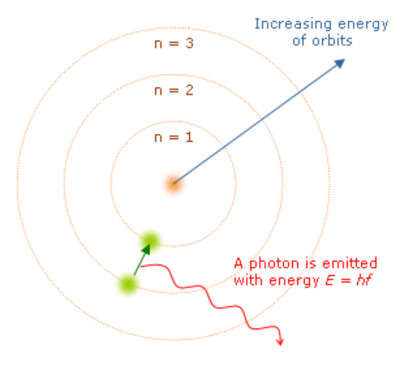
Energy levels of the Bohr atom
Enock Lau Creative Commons Attribution-Share Alike 3.0
Bohr solved Rutherford’s problem with energy loss from the electron, by assuming it just wasn’t a difficulty in these special circumstances.
Bohr still had to decide which orbitals would be allowed. To do this Bohr placed the condition that the angular momentum (mvr) of the electron must be an integral multiple of Planck’s constant divided by 2π.
There are two things I specifically would like to point out.
First, that in a certain sense this condition is equivalent to Planck’s condition on an oscillator.
Secondly, when we use angular momentum to offset the electrostatic attraction between the electron and the proton, the value r represents the distance between the electron and the proton, the same as used in Coulomb’s Law and bringing with it the point charge approximation.